Sutapa Mukherjee, * N.K. Lohiya, † Rahul Pal,‡ M.G. Sharma,‡ and G.P. Talwar*
*International Center for Genetic Engineering and Biotechnology, Aruna Asaf Ali Marg, New Delhi-110067, India. †Department of Zoology, University of Rajasthan, Jaipur-302004, India, and ‡National Institute of Immunology, Aruna Asaf Ali Marg, New Delhi-110067, India
Name and address for correspondence: Dr. G. P. Talwar, Professor of Eminence, International Center for Genetic Engineering and Biotechnology, Aruna Asaf Ali Marg, New Delhi-10067, India. Fax: 91-11-6862316
Sumitted for publication February 13, 1996
Revised March 5, 1996
Accepted for publication March 5, 1996
The use of neem (Azadirachta indica) seed extracts (Praneem) given orally for abrogation of pregnancy in subhuman primates is described. Oral administration of Praneem was initiated after confirmation of pregnancy using Leydig cell bioassay estimating rising levels of chorionic gonadotropin (CG) in the blood from day 25 onwards of the cycle and continued for six days. Termination of pregnancy was observed with the appearance of blood in the vaginal smears and decline in CG and progesterone. Pregnancy continued in the control animals treated with peanut oil at the same dose. The effect was observed in both baboons and bonnet monkeys. The treatment was well tolerated; blood chemistry and liver function tests had normal values. The animals regained their normal cyclicity in the cycles subsequent to Praneem treatment. CONTRACEPTION 1996;53:375-378
KEY WORDS: Azadirachta indica, fertility studies, primates, pregnancy termination
Introduction
Over fifty million abortions are carried out each year around the globe. There is continuing need to develop additional methods, administrable preferably by oral route, to enable the termination of an unwanted pregnancy. A major step in this direction occurred with the introduction of RU486, a progesterone receptor blocking steroid which in combination with prostaglandins could bring about abortions in 96% of cases.1 Plant products have been employed for pregnancy interruption in traditional medicine in many countries. However, objective scientific studies on their efficacy and safety are lacking. We reported recently the abrogation of pregnancy in rats following oral administration of neem (Azadirachta indica) seed extracts;2 the treatment was effective in 100% of animals tested. The treatment was well tolerated and the animals regained fertility in subsequent cycles. The chemical composition of these extracts has been mostly delineated and these contain a number of chemically defined terpenoids and limonoids, besides fats and fatty acids.3 Although common mechanisms exist in reproduction of rodents and primates, there are also distinct differences. As, for example, the pituitary hormones continue to act as gonadotropins in rodents and no gonadotropin of chorionic origin is made in the species; whereas the pituitary is silent in primates and gonadotropic stimulus is provided by chorionic gonadotropin of trophoblastic origin. In order to gauge the potential application of the neem extracts as abortifacients, it was considered appropriate to test their action in subhuman primates. This article describes the results obtained in baboons (Pupio anubis) and bonnet monkeys (Macaca radiata).
Material and Methods
Praneem
Purified neem seed extracts (Praneem) were prepared from taxonomically characterized neem seeds. The oil was extracted in a table-top electrically driven machine (Komet oil expeller, IBG Monforts, Germany), followed by a two-step refining procedure by which the suspended materials were removed. The oil was kept at room temperature (25°C) overnight, the supernatant was decanted and centrifuged at 1500 g for one hour, followed by ultracentrifugation at 65,300 g for another 1hr. The pellet was discarded and the supernatant frozen at 4°C for further study. The preparation was screened to be free of aflatoxins B1, B2, G1, and G2 by TLC analysis on precoated Silica gel G plates using chloroform:isoamyl:alcohol:methanol (90:32:3) as solvent system. Pure aflatoxins from a kit (Sigma Chemical Company) were used as reference standards. The specifications of a batch (PVOO6) of Praneem were: specific gravity 0.905 g/ml; pH 5.7; saponificiation value 206.7; acid value <24; iodine value 24. The free fatty acid composition of the preparation as determined by gas chromatographic analysis was as follows: palmitic acid (19.6%); stearic acid (17.2%); oleic acid (41.2%); linoleic acid (0.82%); and other undetected minor acids (1.65%). The bitter principles include proto-meliacins, meliacins, limonoids, pentanortriterpenoids and norterpenoidal constituents as reported elsewhere.4
Animals
The study was conducted in two primate species, the adult baboons (Papio anubis) and bonnet monkeys (Macaca radiata). They were maintained in semi-natural conditions in the Primate Research Facility of the National Institute of Immunology. Animals of re- productive age only were employed for the study.
Fertility Studies
Female baboons were mated repeatedly with males of proven fertility during the estrous phase indicated by perineal sex swelling. For female bonnets, matings were set from day 8 to day 13 of the cycle.
Sample Collection for Estimation of Chorionic Gonadotropin (CG) and Progesterone Measurement Blood samples were collected on alternate days from day 25 of the cycle for measuring CG by bioassay and twice weekly for measuring progesterone by RIA. Each time, 2 ml of blood was drawn via the femoral vein; the serum was collected and stored at -20°C.
Bioassay for Estimating Chorionic Gonadotropin CG
Chorionic gonadotropin was estimated in the serum samples by Leydig cell bioassay as per method described by Van Damme et a1.5 The method’s basis is that short-term cultures of Leydig cells produce as- sayable quantities of testosterone in the presence of CG and the effect of the hormone is dose-dependent.
Radioimmunoassay for Progesterone
Progesterone levels in the sera samples were determined according to the method described by Brenner et a1.6 employing WHO matched reagents.
Treatment Regime
Neem seed extracts (Praneem) were given orally using a catheter tube after confirming pregnancy by measuring high levels of CG in blood. Animals were anesthetized with Ketamine (0.4 ml Ketamin hydrochloride, Themis Pharmaceutical Ltd, India) before treatment to avoid struggle and stress. Parallel controls received peanut oil.
Results
Pregnancy Termination
The study was conducted in five baboons (Papio anubis) and three bonnet monkeys (Macaca radiata). The pregnancy was monitored by appearance of CG bioactivity in the serum which normally increases over an eight-to-ten-day period before declining. The occurrence of conception was further confirmed by serum progesterone levels which in pregnant animals did not decline. After establishing that the animals were pregnant by these two criteria, they were administered purified neem seed extracts daily for six days by oral feeding tube. Three out of four baboons given the treatment experienced abrogation of pregnancy (Table 1). This was indicated not only by bleeding but also by both progesterone and CG levels falling to near zero levels (Figure 1). The baboon and monkey given an equal amount of peanut oil instead of Praneem continued to maintain pregnancy with sustained progesterone serum levels. Baboon 68, in which no termination of pregnancy took place following administration of purified neem seed extracts, had vomited the oil on the second day of the treatment. It is possible that this baboon did not receive an adequate dose of Praneem.
Figure 1 gives the data and kinetic changes in CG and progesterone profiles of the treated and the control baboons. The treatment was totally effective in the bonnet monkeys tested. The treatment was well tolerated except for one baboon where vomiting took place after the administration of Praneem on day 2 of the treatment. No other behavioral change or alteration in food intake was noted. Blood chemistry and liver function test parameters before and after treatment were not altered (Table 2).
Table 1. Abortifacient action of Praneem given orally in
primates
| Animal # | Treatment and Dose |
Days of Treatment Since LMP |
Day of Onset of Bleeding and Its Duration (Days) |
|
Pan 67 Pan 52 Pan 32 Pan 68* |
Praneem 6 ml for 6 days |
37-42 35-40 37-42 39-44 |
48-50 (3 days) 42-45 (4 days) 44-46 (3 days) Pregnancy continued |
| Pan 63 |
Peanut oil 6 ml for 6 days |
38-43 | Pregnancy continued |
|
MRA 526 MRA 672 |
Praneem 3 ml for 6 days |
43-48 42-47 |
52-54 (3 days) 52-54 (3 days) |
| MRA 638 |
Peanut oil 3 ml for 6 days |
36-41 | Pregnancy continued |
*Vomiting observed on day 2 of treatment.
Figure 1
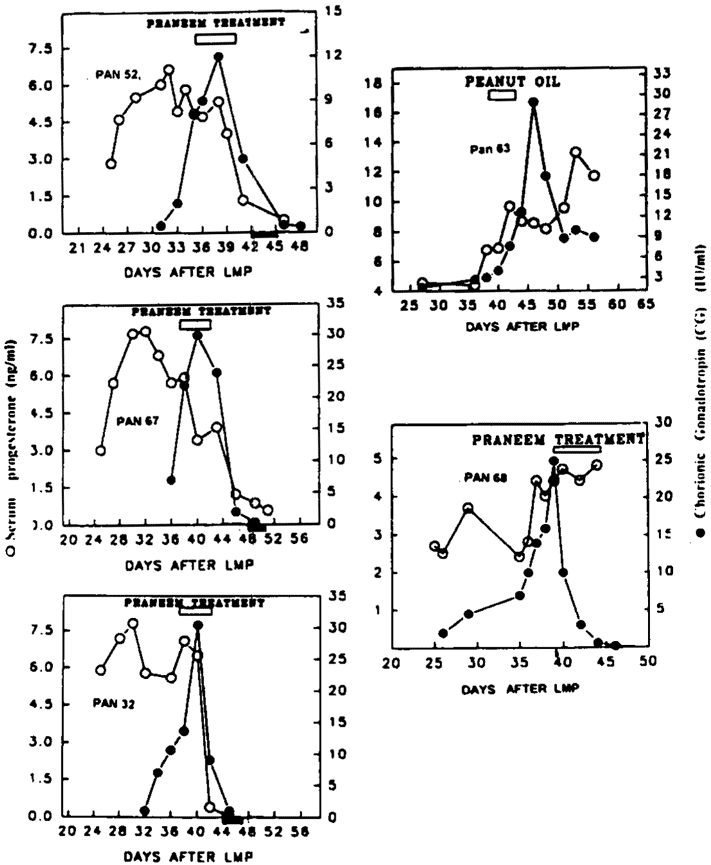
Figure 1. Effect of administration of neem seed extracts (Praneem) given orally in pregnant baboons. Treatment given after confirming CG in blood as illustrated by (). Termination of pregnancy observed by decline in CG (—) and progesterone; (—); () represents vaginal bleeding. Pan 63 shows the control baboon treated with peanut oil; Pan 68, the baboon where abrogation of pregnancy was not observed after Praneem treatment.
Reversibility
The reversibility of the effect of purified neem seed extract (Praneem) was manifested by the observation that baboons whose pregnancy was terminated by this treatment developed perineal sex swelling (due to estrogens) in the subsequent cycles. Normal cyclicity was regained after one irregular cycle. The animals mated with males of proven fertility. On becoming pregnant, the pregnancy proceeded to term. Pups born to these mothers (previously treated with Praneem) were normal. Figure 2 gives the case history of two such baboons. The treatment had no apparent residual effects on reproductive functions.
Effect of Praneem Oral Treatment on Progesterone
Data in Figure 1 show that soon after institution of the treatment with Praneem, progesterone decline commences. On the other hand, no consistent kinetic correlation is seen with CG levels. One could, thus, hypothesize that the treatment may be causing the lysis of the corpus luteum, the ovaries being the source of progesterone in these species at this stage of pregnancy. To test this hypothesis, normally cycling baboons were given oral treatment with Praneem for six days at the same dose from day 18 to 23 of the cycle. However, no shortening of the menstrual cycles was noted. Thus, Praneem does not appear to impair the corpus luteum function of the nonpregnant female baboon. The mechanism by which abrogation is caused may be similar to those identified during the previous study2 in rodents.
Discussion
This study demonstrates that Praneem administered orally for six days after confirming pregnancy by the rising levels of CG in the blood, brought about termination of pregnancy; peanut oil given by the same route at the same dose did not show this effect. The effect was reversible and fertility was regained in the cycles subsequent to Praneem treatment. The treatment was well tolerated with no residual effect compromising the future fertility of the animals.
Table 2. Hematological and clinical chemistry parameters as studied in baboons with Praneem
| Praneem (n=3) | |||
| Parameter | Before Treatment (Mean + SEM) |
After Treatment (Mean + SEM) |
|
| Hb (g%) | 11.7 + 0.26 | 11.6 + 0.1 | |
| TLC/mm3 (thousands) | 7.3 + 0.44 | 7.0 + 0.26 | |
| DLC (%) Neutrophils | 55.3 + 2.02 | 56.0 + 2.3 | |
| Lymphocytes | 42.3 + 2.02 | 41.3 + 2.9 | |
| Moocytes | 1.0 + 0.01 | 1.3 + 0.33 | |
| Eosinophils | 1.3 + 0.33 | 1.3 + 0.33 | |
| Bilirubin (mg%) | 0.43 + 0.03 | .46 + 0.06 | |
| SGPT (IU/lit) | 22.0 + 1.15 | 22.6 + 1.3 | |
| SGOT (IU/lit) | 25.3 + 1.3 | 26.0 + 1.15 | |
| Urea (mg%) | 27.3 + 0.33 | 29.0 + 0.01 | |
| Creatinine (mg%) | 1.06 + 0.03 | 1.1 + 0.04 | |
| Glucose (mg%) | 64.3 + 2.3 | 63.0 + 2.51 | |
| Total | 7.1 + 0.2 | 7.23 + 0.14 | |
| Albumin | 4.0 + 0.17 | 4.16 + 0.08 | |
| Globulin | 3.1 + 0.033 | 3.13 + 0.06 | |
n = number of baboons; TLC, total leucocyte count; DLC, differential leucocyte count; SGPT, serum glutamate oxaltate transaminase; SGPT, serum glutamate pyruvate transaminase.
Figure 2
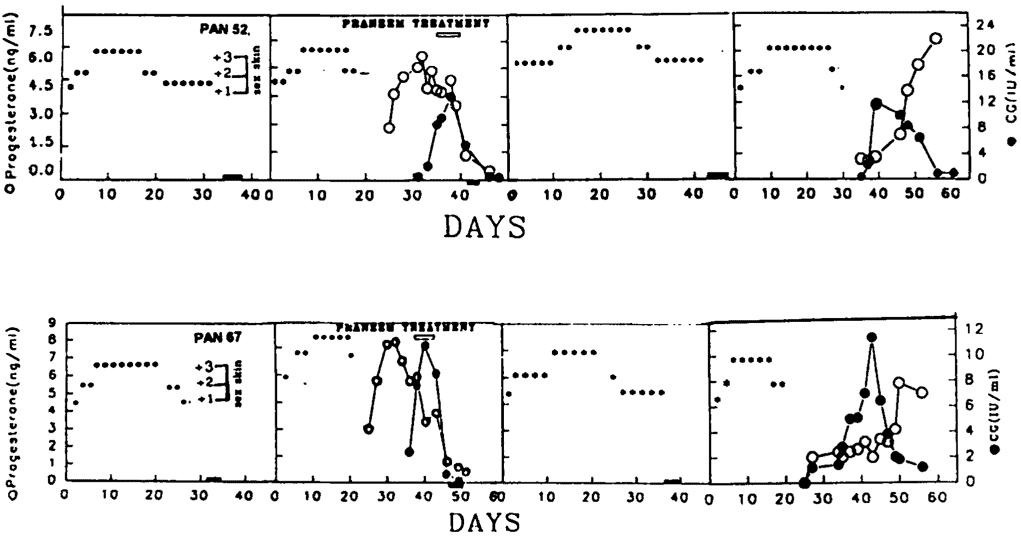
Figure 2. Perineal sex swelling (***) pattern of baboons Pan 52 and Pan 67 and after treatment with Praneem in pregnant cycle. Both animals regained fertility in a subsequent cycle.
Neem (Azadirachta induce) extracts have strong immunomodulatory properties.7 Evidence has been gathered to show that immunological mechanisms play a role in maintenance of pregnancy.8 Cytokines secreted by T-helper 1 cells, i.e., gamma interferon and TNF alpha, have detrimental effects on fetal survival, whereas cytokines IL-3 and GM-CSF help in gestation.9 Our previous work has shown that oral administration of purified neem seed extracts (Praneem) caused an elevation of both the immunoreactive and bioactive TNF alpha and gamma interferon in the sera. The draining mesenteric lymph node cells synthesized and secreted these cytokines and Th 1 type of cytokines were also present in the fetoplacental cultures. These transitory changes were presumably the basis of termination of pregnancy in rodents. Similar mechanisms may be responsible for termination of pregnancy in primates following ingestion of the neem seed extracts.
Acknowledgments
This work was supported by research grants of the Rockefeller Foundation and South-to-South collaboration in Reproductive Health. We would like to thank Dr. Om Singh and Dr. S. Majumdar for helpful suggestions.
References
1. Silvestre L, Dubois C, Renault M, Rezvani Y, Baulieu EE, Ulmann A. Voluntary interruption of pregnancy with mifepristone large-scale 645-8. (RU486) and a prostaglandin analog: A large-scale French experience. New Engl J Med 1990; 322: 645-8.
2. Mukherjee S, Talwar GP. Termination of pregnancy in rodents by oral administration of Praneem, a purified enema seed extract. Am J Reprod Immunol 1996; 35: 51-6.
3. Devkumar C, Sukh D. Chemistry. In: Randhawa NS, Parmar BS, eds. Neem Research and Development. India: Society of Pesticide Science, 1993: 63-97.
4. Siddiqui SS, Mahmood T, Siddiqui BS, Faizi S. Nonterpenoidal constituents from A. indica. Planta Medica 1988; 54: 457-62.
5. Van Damme MP, Robertson DN, Diczfalusy E. An improved in vitro bioassay method for measuring luteinizing hormone (LH) activity using cell preparations. Acta Endocrinol (kbh) 1974; 77: 655-71.
6. Brenner PF, Guerrero R, Cekan Z, Diczfalusy E. Radioimmunoassay method for sex steroids in human plasma. Steroids 1973; 22: 775.
7. Labadie RP, Van der Nat JM, Simons JM, Kroes BH. An ethanopharmacognistic approach to the search for immunomodulators of plant origin. Planta Medica 1989; 55: 339-48.
8. Wegmann TG, Lin H, Guilbert L, Mossmann TH. Bidirectional cytokines in the maternofetal relationship: successful allopregnancy is Th2 phenomenon. Immunology Today 1993; 14:353-8.
9. Chaouat G, Menu E, Clark DA, Dy M, Minkowski M, Wegmann TG. Control of fetal survival in CBA x DBA/2 mice by lymphokine therapy. J Reprod Fertil 1990; 89: 447-58.